Contextual nature of AUC
The area under the receiver operating characteristic (AUC) is arguably among the most frequently used measures of classification performance. Unlike other common measures like sensitivity, specificity, or accuracy, the AUC does not require (often arbitrary) thresholds. It also lends itself to a very simple and intuitive interpretation: a models AUC equals the probability that, for any randomly chosen pair with and without the outcome, the observation with the outcome is assigned a higher risk score by the model than the observation without the outcome, or
where
The ubiquitous use of AUC isn’t without controversy (which, like so many things these days, spilled over into Twitter). Regularly voiced criticisms of AUC — and the closely linked receiver operating characteristic (ROC) curve — include its indifference to class imbalance and extreme observations.
In this post, I want to take a closer look at a feature of AUC that — at least in my experience — is often overlooked when evaluating models in an external test set: the dependence of the AUC on the distribution of variables in the test set. We will see that this can lead to considerable changes in estimated AUC even if our model is actually correct, and make it harder to disentangle changes in performance due to model misspecification from changes in performance due to differences between development and test sets.
Who this post is for
Here’s what I assume you to know:
- You’re familiar with R and the tidyverse.
- You know a little bit about fitting and evaluating linear regression models.
We will use the following R packages throughout this post:
library(MASS)
library(tidyverse)
library(tidymodels)Generating some dummy data
Let’s get started by simulating some fake medical data for 100,000 patients. Assume we are interested in predicting their probability of death (=outcome). We use the patients’ sex (binary) and three continuous measurements — age, blood pressure , and cholesterol — to do so. In this fake data set, being female has a strong protective effect (odds ratio = exp(-2) = 0.14) and all other variables have a moderate effect (odds ratio per standard deviation = exp(0.3) = 1.35). Any influence by other, unmeasured factors is simulated by drawing from a Bernoulli distribution with a probability defined by sex, age, blood pressure, and cholesterol.
set.seed(42)
# Set number of rows and predictors
n <- 100000
p <- 3
# Simulate an additional binary predictor (e.g., sex)
sex <- rep(0:1, each = n %/% 2)
# Simulate multivariate normal predictors (e.g., age, blood pressure,
# cholesterol)
mu <- rep(0, p)
Sigma <- 0.8 * diag(p) + 0.2 * matrix(1, p, p)
other_covars <- MASS::mvrnorm(n, mu, Sigma)
colnames(other_covars) <- c("age", "bp", "chol")
# Simulate binary outcome (e.g., death)
logistic <- function(x) 1 / (1 + exp(-x))
betas <- c(0.8, -2, 0.3, 0.3, 0.3)
lp <- cbind(1, sex, other_covars) %*% betas
death <- rbinom(n, 1, logistic(lp))
# Make into a data.frame and split into a training set (first half) and
# a biased test set (rows in the second half X * beta > 0)
data <- as_tibble(other_covars)
data$sex <- factor(sex, 0:1, c("male", "female"))
data$pred_risk <- as.vector(logistic(lp))
data$death <- factor(death, 0:1, c("no", "yes"))
data$id <- 1:nrow(data)Estimating predictive performance
Now that we have some data, we can evaluate how well our model is able to
predict each patient’s risk of death. To make our lives as simple as possible,
we assume that we were able to divine the true effects of each of our variables,
i.e., we know that the data is generated via a logistic regression model with
auc <- function(data) {
yardstick::roc_auc(data, death, pred_risk, event_level = "second")
}
data %>% auc()## # A tibble: 1 × 3
## .metric .estimator .estimate
## <chr> <chr> <dbl>
## 1 roc_auc binary 0.785Notably, this is a summary measure that depends on the entire data set
men <- data %>% filter(sex == "male")
men %>% auc()## # A tibble: 1 × 3
## .metric .estimator .estimate
## <chr> <chr> <dbl>
## 1 roc_auc binary 0.662women <- data %>% filter(sex == "female")
women %>% auc()## # A tibble: 1 × 3
## .metric .estimator .estimate
## <chr> <chr> <dbl>
## 1 roc_auc binary 0.665In each subset, the AUC dropped from 0.78 to around 0.66. This perhaps isn’t too surprising, given that sex was a strong predictor of death. However, remember that the model coefficients and hence the predicted risk for each individual patient — i.e., how “good” that prediction is for that patient — remain unchanged. We merely changed the set of patients that we included in the evaluation. Although this might be obvious, I believe this is an important point to highlight.
Looking at the distribution of risks in the total data and by sex might provide some further intuition for this finding. Predicted risks for both those who did and did not die are clearly bimodal in the total population around the average risk for men and women (Figure 1). Even so, there is good separation between them. The majority of patients who died (red curve) had a predicted risk >50%. Vice versa, the majority of patients who remained alive (green curve) had a risk <50%. Looking at the risks of men and women separately, however, we can see that most men had a high and most women a low predicted risk (Figure 2). There is much less separation between the red and green curves, as any differences among for example the men is entirely due to moderate effects of our simulated continuous covariates.
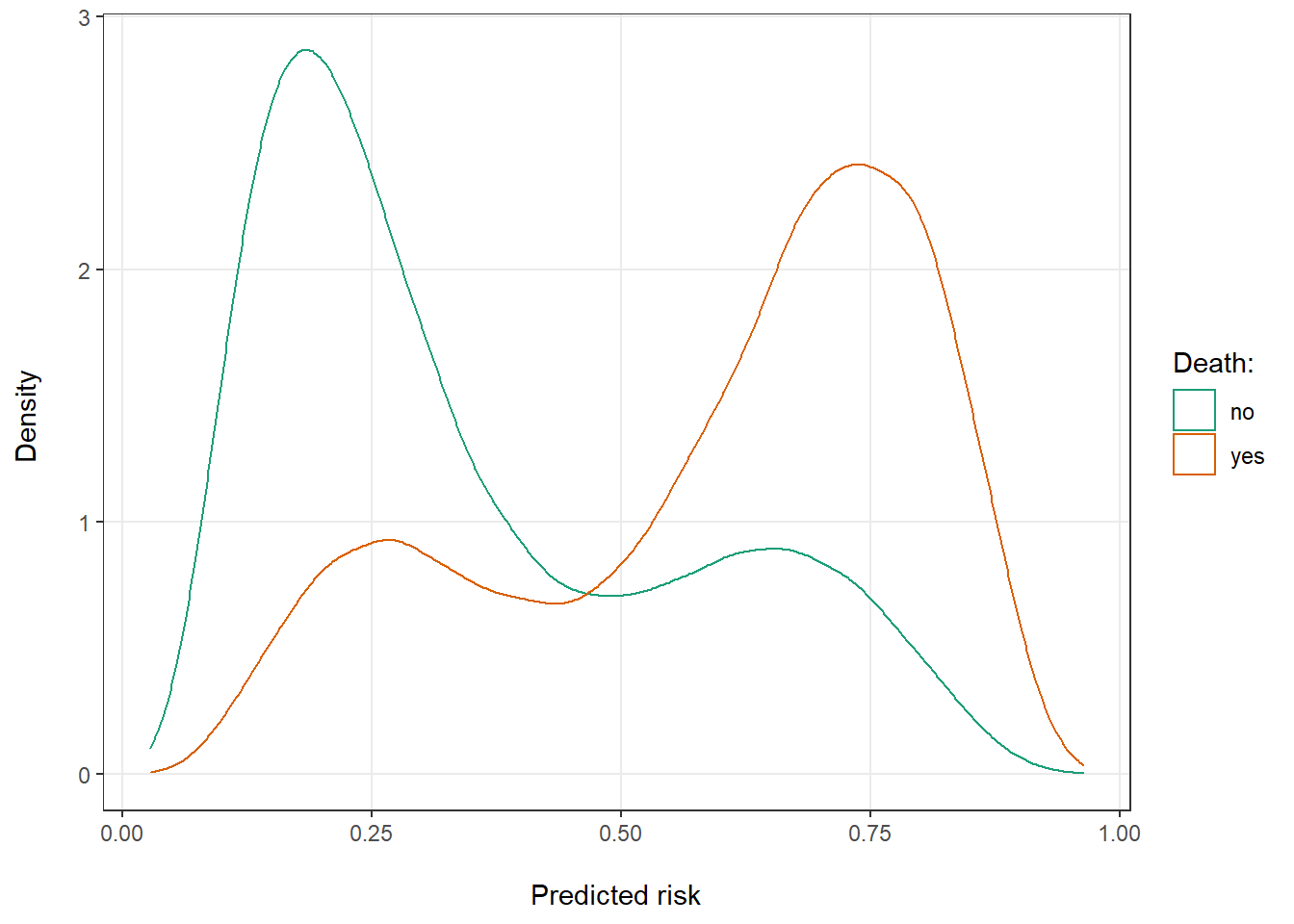
Figure 1: Distribution of predicted risk of death for patients that ultimately did and did not die.
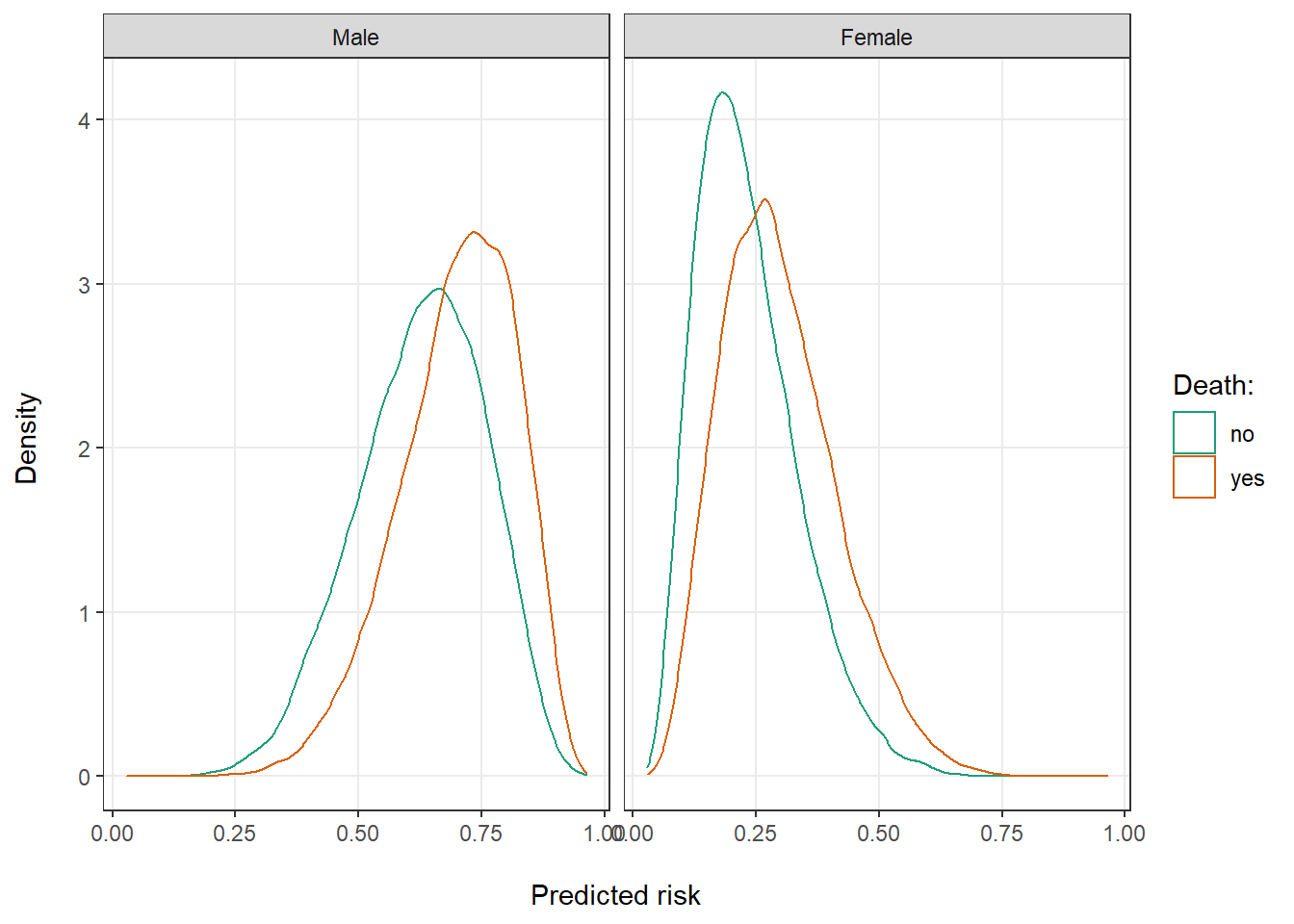
Figure 2: Distribution of predicted risk of death for by sex.
So far, we have looked at two extremes: the entire data set, in which sex was perfectly balanced, and two completely separated subsets with only men or only women. Let’s see what would happen if we gradually reduce the number of men in our evaluation population. We can see that estimated performance drops as we remove more and more men from the data set (Figure 3), particularly at the right side of the graph when there are only few men left and there is an increasing sex imbalance.
auc_biased <- function(data, p_men_remove) {
n_men <- sum(data$sex == "male")
n_exclude <- floor(n_men * p_men_remove)
data %>%
slice(-(1:n_exclude)) %>%
auc()
}
p_men_remove <- seq(0, 1, by = 0.01)
auc_shift <- p_men_remove %>%
map_dfr(auc_biased, data = data) %>%
mutate(p_men_remove = p_men_remove)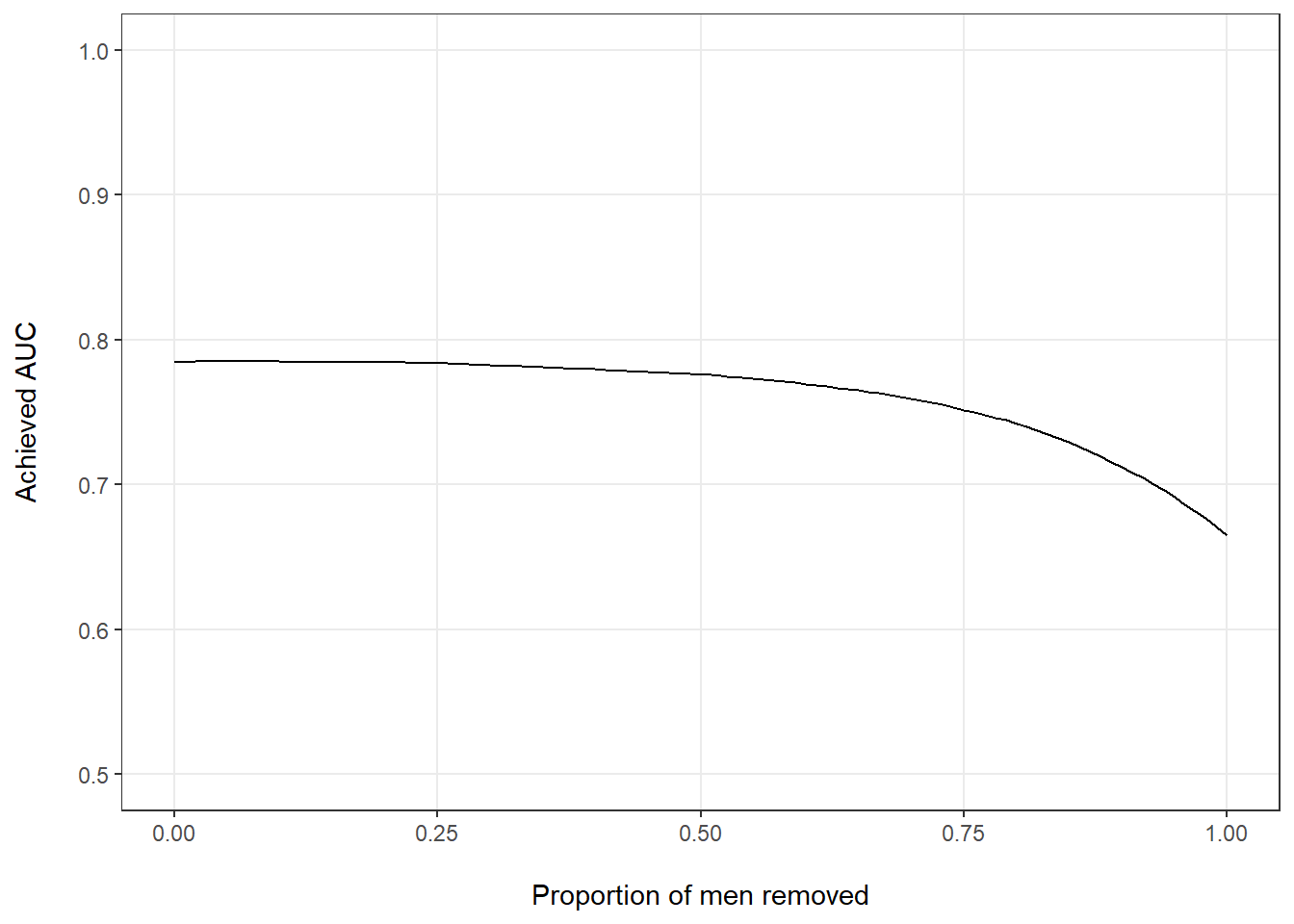
Figure 3: Estimated AUC by proportion of men removed from the full data set.
Difficulty to distinguish model misspecification
What we have seen so far is particularly problematic in the interpretation of
external model validation, i.e., when we test a model that was developed in one
set of patients (and potentially overfit to that population) in another patient
population in order to estimate the model’s likely future performance. This is
because in most real world cases, it isn’t quite as straightforward to quantify
the difference between the development population and the evaluation cohort.
Since we also usually don’t know the true model parameters (or even the true
model class), it is difficult to disentangle the effects of population makeup
from the effects of model misspecification. Let’s assume for example that —
unlike earlier — we don’t know the exact model parameters and instead needed
to estimate them from a prior development data set. As a result, we obtained
biased_betas <- c(0.4, -1, 0.3, -0.2, 0.5)
alt_risk <- logistic(cbind(1, sex, other_covars) %*% biased_betas) %>%
as.vector()
data %>%
mutate(pred_risk = alt_risk) %>%
auc_biased(0.5)## # A tibble: 1 × 3
## .metric .estimator .estimate
## <chr> <chr> <dbl>
## 1 roc_auc binary 0.722Again, the estimated model performance dropped. However, how much of the drop
was due to our biased estimate
Takeaway
If there is one takeaway from this post it is that external validations of predictive models mustn’t solely report on differences in AUC but also need to comment on the comparability of development and test sets used. Such a discussion is warranted irrespective of whether the performance remained the same, dropped, or even increased in the test set. Only by discussion — and ideally even quantifying — differences between the data sets can the reader fully assess the evidence for retained model performance and judge its likely value in the future.